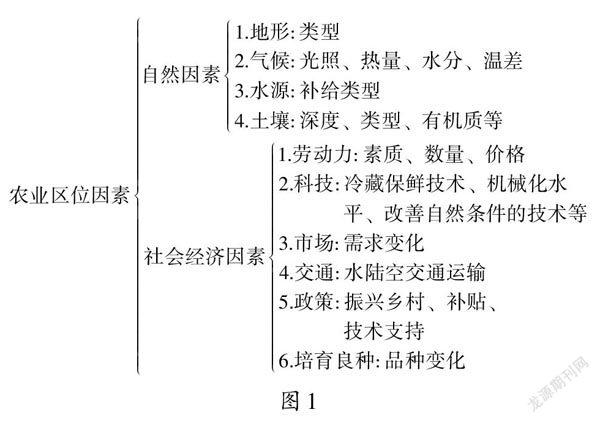
【www.zhangdahai.com--其他范文】
Hidyh Mnn, A.B. Noor Hidyti, Nur Ain Lyn, Adnn Amin-Sfwn,Hongyu M, Nor Azmn Ksn,d, Mhd Ikhwnuddin,d
a Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu,Malaysia
b Institute of Biological Science, Faculty of Science, University of Malaya, Kuala Lumpur, 50603, Malaysia
c Guangdong Provincial Key Laboratory of Marine Biotechnology, Shantou University, Shantou, 515063, China
d STU-UMT Joint Shellfish Research Laboratory, Shantou University, Shantou, 515063, China
Keywords:UV-Length Irradiated sperm Fertilized eggs Hatching rate Survival rate Gynogens
ABSTRACT

Fig. 1. Meiotic gynogenesis process using UV irradiated sperm and cold shock to prevent extrusion of second polar body for production of diploid gynogens.
Nowadays, monosex culture has become a common practice in aquaculture due to the various size of female and male crustacean or known as sexual dimorphism (Guerrero-Tortolero & Campos-Ramos,2018, chap. 36; Hidayah et al., 2012). In fact, female was identified economically attractive because of it larger in size and has faster growth rate compared to culture of all male penaeids shrimp such as Litopenaeusvannamei, P. japonicus, P. monodon, P. indicus, and Fenneropenaeus chinensis(Beardmore et al., 2001; Guerrero-Tortolero & Campos-Ramos,2018, chap. 36; Hidayah et al., 2013; Ikhwanuddin et al., 2019; Rodgers et al., 2006). One of the selected practices for monosex culture is through gynogenesis induction that has been applied in last few years in a number of fishes. Up to date, artificial induction of gynogenesis has been applied in many types of fish species such as inPseudosciaena crocea(Komen & Thorgaard, 2007; Xu et al., 2007). Gynogenesis was identified as one of the potential technique in order to produce all female larvae offspring in marine shrimp species (Hidayati et al., 2014). For example,Emefe and Sorhue (2015) applied gynogenesis to control fish population and to increase the profit in aquaculture forClaria gariepinusfish species.Basically, to induce gynogenesis, unfertilized eggs were fertilized with irradiated sperm of same species and treated with temperature such as cold shock or by chemical shock by using cytochalasin-B (Cai & Feng,1993). According to Stanley and Sneed (1974), gynogenesis is a process which is related to parthenogenesis, however the development of the ovum after the sperm penetrations is without syngamy and it’s occurred naturally in a few fishes. Through gynogenesis technique, the development of the larvae only contains the maternal DNA due to activation of an egg with the irradiated sperm. In the gynogenesis process, the egg is developing without the DNA contribution from the paternal due to the application with the irradiated sperms. Cytological examination technique in the fertilized eggs and through the observation of the egg was applied to identify the percentage of gynogenesis successful in the Chinese shrimp (Cai & Feng, 1993). Basically, the purpose of gynogenesis is to eliminate the male inheritance by destroying the DNA in the spermatozoa through UV irradiation on the sperms and also to restore diploidy to the ovum by disturbing the meiosis or the cell cleavage process (Stanley & Sneed, 1974). Bennan et al. (1997) during artificial induction of gynogenesis in Chinese shrimp applied 365 nm UV length to irradiate the sperm for 5–8 s to produce DNA damage in the sperm. To successfully produce artificial gynogenesis, firstly the genetic inactivation of the sperm should be carried out and secondly the successfully turn out of the gynogen genetic to diploid. Usually, to inactivate the paternal chromosome is by exposing the sperm to the chemical or radiation for examples the X-rays, y-rays or the UV penetration. Wu et al.(1991) explained the spontaneous gynogenesis that being produce usually in lower number around 0.2% in the fish carp and the viability of a large number of induced eggs can survive the generation of a few diploid individually. The gynogens progeny can be produced artificially by inhibiting the extrusion of second polar body by inhibiting the first mitotic division in the embryo. This process can be achieved by treatment with high or low temperature shock or either by chemical shock such as colchicines. Gynogenesis through mitotic interference will produce homozygous diploids larvae. The percentage of gynogenetic diploid induced through temperature shock was identified successful to produce around 38%–80% gynogens and the offspring produced might be sterile due to chromosome deletion or breakage (Kocher & Kole,2008, p. 180; Streisinger et al., 1981). Due to the important of the study in gynogenesis technique in aquatic species, this review paper was introduced to combine all the technique and protocol for the application of gynogenesis with the optimal and correct dosage. This review paper also brings to the future of gynogen offsprings of all female type by following the established technique proposed in the previous study in this review paper.

Fig. 2. Mitotic gynogenesis process using UV irradiated sperm and cold shock applied after extrusion of second polar body for production of diploid gynogens.
Gynogenesis is a special and established techniques where the offspring chromosome is inherited from the mother side. Gynogenesis is also known as a gene manipulation technique to produce all female type of fish (Emefe & Sorhue., 2014). As stated by Azhaguchamy (2013),gynogenesis is a technique to produce all female population without genetic contribution of male gametes due to the fused up with the irradiated sperms. There are two types of gynogenesis process which are meiotic gynogenesis and mitotic gynogenesis that can be produced by artificial diploidization of the maternal chromosome (Hussain, 2004).The induction of meiotic gynogenesis was done by fertilized the eggs with UV irradiated sperm and exposed it to shock either by physical such as cold shock or chemical shock to suppress the anaphase stage of second meiotic division (Hussain, 2004). According to Li et al. (2018), meiotic gynogenesis was artificially induced by activating the developed egg with the UV irradiated heterogenous sperm after prevention of second polar body extrusion by cold shock. Hidayah et al. (2013) identified the extrusion of second polar body at 8–15 min after spawning and 10 min after spawning was chosen to apply the shock to prevent the polar body extrusion to induce triploidy inPenaeus merguiensisshrimp. Nan’er,Feng, Yafu, & Bennan, 1995 proposed that the procedure of the retention of second polar body by cold shock or cytochalasin B shocks were same as commonly applied in the triploidy procedure. Through the induction of mitotic gynogenesis, it was done by the inhibition of first cleavage at the mitotic division (Hussain, 2004) and this process was carried out through application of physical shock such as cold shock or pressure shock to the mitotic process. Li et al. (2018) and Yamamoto(1999) discovered that the mitotic gynogenesis can produce double diploid by blocking the first mitotic division using external shock. They found out from these two gynogenesis technique meiotic gynogenesis identified generates fewer homozygous diploids through chromosome synapses but give higher result of survivability and fertility off the offspring produced (Li et al., 2018). The two types of gynogenesis techniques of meiotic and mitotic were shown in Fig. 1 and Fig. 2 on how the process of gynogenesis conducted respectively. Attempts have been made to induce gynogenesis diploid in European loach by applying thermal shock or hydrostatic pressure shock to the egg after the fertilization with irradiated spermatozoa using gamma or UV rays (Suzuki et al., 1985). It was also identified that sometimes, spontaneous gynogenesis occurred in some telosts, amphibians and also in reptilian(Komen & Thorgaard, 2007; Miao et al., 2014). Currently, gynogenesis and hormone sex reversal are used in aquaculture to generate monosex populations which will help increase the economic income and enhancing the aquaculture productions (Azhaguchamy, 2013). During gynogenesis application, the sperms which contain the male genetic material were destroyed by using either Gamma (y), X or by Ultraviolet(UV) rays. Then these sperms were directly applied to fertilize the eggs cell during gynogenesis process. There are varieties of shock can be used include, heat shock, cold shock, hydrostatic pressure, chemical shock such as Colchicine and Cytochalasin-B in order to retain the second polar body to produce gynogens offspring (Ozdemir & Aygul, 2017). The application of the shock is to allow the egg to proceed with normal developments which contains two sets of chromosomes in the gynogen(Lutz, 2008, pp. 120-140). Artificial induction of gynogenesis is also a technique to manipulate the chromosome set and is wisely applied to begin the genetic mechanisms of sex in the fish. This technique also can be used to establish homogametic XX male or WW female broodstock with a sex chromosome system of female XX/XY or male homogametic ZZ/ZW respectively (Pandian & Koteeswaran, 1998; Devlin & Nagahama, 2002). In gynogenesis, it’s involve two important step, the first step is the elimination of the paternal genetic material by using the irradiated sperm and the second step involve the diploidization of the gynogenetic haploid by a shock given to retain the second polar body which is meiotic gynogenesis (Azhaguchamy, 2013).
Over the world, penaeid shrimp has been known by the entrepreneurs and aquaculturist to be the important culture species due to its higher growth rate and tolerance to the varies environment (Hidayah et al., 2013; Seneetha et al., 2009). Basically, penaeid shrimp is animportant culture species which carry on external fertilization (Malecha& Hedgecock, 1989). Due to its external fertilization, the chromosome manipulation can be induced in the penaeid shrimp as an alternative to sustain production. In prawn and shrimp, they have been identified with WZ/ZZ sex determination system where female were identified hetero-gamety where female is the one that determines sex and for male sex differentiation is controlled by the adrogenic gland of the shrimp(Guerrero-Tortolero & Campos-Ramos, 2018, chap. 36). To meet the higher demand, advantages of increasing sterility are exploited during commercial growing to obtain suitable size for commercialize shrimp(Hidayah et al., 2013). Induction of triploidy, gynogenesis, inter species hybridization can be applied in this type of penaeid shrimp (Campos--Ramos et al., 2006). From the previous study, there is very scanty research has been conducted on the gynogenesis induction in shrimp.One of its which was Bennan et al. (1997) induced gynogenesis in Chinese shrimp,Penaeus chinensisand identified that sperm irradiated at 365 nm ultraviolet (UV) can fertilized the egg; however the activated eggs died in the early stages of embryonic development and could not hatch. The method 365 nm of the UV irradiated (UV) for 5–8 s can be applied to get the genetic inactivation of the sperm to carry on the gynogenesis induction (Bennan et al., 1997). Hidayati et al. (2014) applied UV irradiated dosage at 254 nm and 365 nm from 20 to 80 s for the initial development of gynogenesis in Banana shrimp,Fenneropenaeus merguiensisand identified sperms abnormalities with damage on malformed bodies also with short or missing spike on the irradiated sperms.However, by applying the 365 nm UV the sperm have a chemical changing which call chromosomal denaturation (Bennan et al., 1997).Nan’er, Feng, & Gall, 1991 induced gynogenesis in Chinese shrimp,Penaeus orientalis kishinouyeto produce all female shrimp populations.The unfertilized eggs were fertilized with irradiated sperm of same species and then were treated with cold shock or cytochalasin B to induce gynogens progeny. This gynogenesis process successfully produced 14.9% and 37.2% of haploid gynogenetic embryos. Through the cytological experiment, study found that the irradiated male nucleus fails to fuse with the female shrimp nucleus to form a zygote (Nan’er,Feng, & Gall, 1991). Other study conducted by Nan’er, Feng, Yafu, &Bennan, 1995 onPenaues chinensisto induce gynogenesis using 30 W of 105 UV irradiation successfully produced 14.0% and 37.2% hatched diploid gynogenetic embryos and identified that the most efficient sperms irradiation methods is the direct irradiation of sperm from the seminal receptacles of the female. Artificial induction of gynogenesis techniques induced in shrimps was shown in Table 1.

Table 1 Artificial induction of gynogenesis techniques induced in shrimps.
The higher demands on the animals protein supply focused on developing new method for increasing the fisheries production (Chakraborty et al., 2006). The new technique currently known as gynogenesis which identified promotes an opportunity to produce higher production of fish stock. This technique has been successfully applied in manycommercial fish species such as in carps (Komen et al., 1991), in catfish(Goudie et al., 1995), in tilapia (Hussain, 1995) and in salmonids(Purdom et al., 1985) for the selective breeding purposes. Thorgaard and Allen (1987) found out several reports described the techniques to induce polyploidy and uniparental chromosome inheritance which applying gynogenesis and androgenesis technique in fish. According to Colombo et al. (1995) the technique of polyploidy and gynogenesis were applied in fish culture in order to produce inbred line and also for the production of monosex or sterile populations. Gynogenesis has been introduced with different purpose in genetic and breeding studies and the previous use of gynogenesis in the production of inbred lines in fishes (Chakraborty et al., 2006; Purdon, 1983). According to Carter et al. (1991), the early work on the gynogenesis in fish only concentrated on the suppression of the second division of meiosis to induce diploidization of the eggs fertilized with spermatozoa that already inactivated using gamma or UV irradiation. Gynogenesis have been successfully induce in number of fishes such as in grass carp (Shelton, 1987), in sliver carp (Chakraborty et al., 2006; Mirza and Shelton, 1988), in tilapia,Oreochromis niloticus, (Muller-Belecke & Horstgen-Schwark, 1995) and in zebrafish,Danio rerio(Odzemir & Aygul, (2017). Peruzzi and Chatain(2000) in their study applied pressure shock and cold shocks to produce gynogenesis and triploid in the European sea bass,Dicentrarchus labraxspecies. They obtained meiogenesis by fertilizing eggs at UV irradiated at 32, 000 erg/mm2. The optimum treatment they obtained for cold shock is at 0–1◦C for 15–20 min and 8500 psi of pressure shock for 2 min at 6 mic a.f (Peruzzi & Chatain, 2000). Gheyas et al. (2001) applied cold shock treatment to produce diploid gynogenesis in Stinging catfish,Heteropneustes fossilisand discovered that the optimum cold shock at 2◦C for 10–15 min was successfully produced the maximum percentage of gynogen progeny. The finding of the project also expected to be the platform for the production of all diploid gynogens and also the technique to produce of monosex female populations in stinging catfish.Artificial induction of gynogenesis techniques induced in fishes was shown in Table 2.

Table 2 Artificial induction of gynogenesis techniques induced in fishes.

Table 3 Artificial Induction of gynogenesis technique induced in molluscs.
In the molluscs, gynogenetic offspring can be successfully produced by retention of the second polar body and has been applied inHaliotis discus hannai,Crassostrea gigas,Mulinia lateralis,Mytilus edulis,Mytilus galloprovincialisand inChlamys farrerispecies (Li & Kijima, 2005). Gynogenesis induction has been successfully applied in few species of bivalve and gastropod and has been reported to survive only 10 and 6 months as stated in previous study for CrassotreagigasandHaliotis discusspecies (Guo et al., 1993; Pan et al., 2004). The Zhilong scallop,Chlamys farreriwas known as important seafood in Northern of China. There is many researches carried out on scallop and polyploidy that successfully produced in these recent year. However, there is still no study has been conducted on gynogenesis of this scallop species (Pan et al., 2004).Study carried on mussels,Mytilus galloprovincialiswith 2 min irradiation of 62 erg/mm2was identified successfully produced gynogenetic offspring (Scarpa et al., 1994). With the application of 30–60s UV irradiation on the sperm with 256 erg/mm2 was successfully induced haploid gynogenesis on the scallop,Chlamys farreri(Pan et al., 2004).Guo et al. (1993) successfully applied gynogenesis technique in Pacific oyster using ultraviolet irradiation on the sperms at 253.7 nm for 5–6 min. Pan et al. (2004) examined the effects of ultraviolet (UV) irradiation on the genetic inactivation and structure of the sperms of scallopChlamys farrerriand found out that UV irradiation of sperms for 30 s at the UV intensity of 256 erg/mm2was the optimum dosage to produce haploid gynogenesis on the observations on the rate of cleaved eggs,chromosome constitutions and flow cytometry analysis on the gynogens larvae. Pan et al. (2004) also discovered the presence of “Hertwing effect” in the gynogen larvae ofChlamys farreriand also clear destruction of the acrosome, cytoplasmic membrane and flagellum of the UV irradiated sperms. Artificial induction of gynogenesis techniques induced in molluscs was shown in Table 3.
Gynogenesis is produced by inhibiting the second meiotic division in many fish species. However, in shellfish it is only can be achieved in a few species such as in abalone and oyster. This is the reason from the high mortality during embryo stage or fertilization stage due to the injury or ruptured of the spermatozoa during UV irradiation (Guo et al.,1993). Study conducted by Bennan et al. (1997) has identified damage to the sperm due to the chemical change on the chromosome denaturation during the UV irradiation process to induce gynogenesis. In addition, for the mitotic gynogens progeny, it was identified have a very low survival rates due to the side effect of the treatment or by the deleterious of recessive genes and usually this type of gynogens progeny exhibited decrease in reproductive capacity. The mitotic gynogens also identified produced low quality of eggs and was not able to produce progeny (Arai, 2000). Samonte-Padilla et al. (2011) through the identification of ploidy level by flow cytometry has identified the haploid gynogenetic embryo shows abnormalities meanwhile the diploids gynogens were developed normally.
In gynogenesis, the male broodstock does not contributed to the genetic material to the progeny and its only contains maternal genome as the sperm only act as the stimulator for the egg to develop (Ottera et al., 2011). From the previous study conducted identified that the diploid gynogens fish were grow faster and built stronger disease resistance than the haploid progeny (Samonte-Padilla et al., 2011). In addition, other study conducted by Ozdemir & Ekici (2017) identified ruptured chromosome fragments from the karyotyping analysis conducted in the haploid gynogenesis embryo of Zebra fish,Danio rerio.Meanwhile, in three-spined stickleback fish, diploid gynogenesis successfully identified by microsatellite DNA analysis that exposed material inheritance in the gynogens progeny (Samonte-Padilla et al., 2011).Through the microsatellite DNA analysis also 97%–100% of the samples were identified heterozygous for the maternal alleles which suggest thatthe majority of the gynogens progeny are meiotic diploids (Samonte--Padilla et al., 2011). Usually for the diploid mitotic gynogens, all the gene loci will be homozygous due to the diploidization occurrence by the doubling chromosome in somatic division. Meanwhile, for the meiotic gynogens which produced by inhibiting of second polar body,the gene loci should be recombinant genotypes due to high rate of gene centromere recombination (Pandian & Koteeswaran, 1998). Thus, the progeny from the female with heterozygous gene loci it should have heterozygous genotypes in the loci. The obvious genetic differences between meiotic and mitotic gynogens are the gene centromere recombination rate, coefficient of inbreeding and variability of quantitative traits (Arai, 2000). The similarities and differentiation between the gynogenesis manipulation among fishes, molluscs and shrimp were shown in Table 4.

Table 4 Similarities and differentiation between the gynogenesis among fishes, molluscs and shrimps.
Gynogenesis technique was proven to be one of the established techniques to produce all type of female diploid offspring in aquatic animals. Other than triploids, gynogenesis technique also proved as one of the techniques for monosex culture for enhancing the aquaculture production. In fishes, this technique has been successfully applied to produce all type of female fish. UV irradiated of the sperms were fused with the unfertilized egg to produce gynogens larvae and the procedure were continued by cold shock, chemical shock or pressure shock to retain the second polar body from extrude out of to induce diploid gynogens. Overall, gynogenesis technique was identified as successful approach and it can be applied in fishes, marine shrimps, and molluscs species such as oyster, abalone and scallops in order to produce all female gynogen products which further enhance and increases the aquaculture yield production. Further study should be focused on the improvement of the gynogenesis technique to increase the hatching rate,survival rate and quality of the gynogenesis larvae in the future.
Ethical approval
“All applicable international, national, and institutional guidelines for the care and use of animals were followed by the authors.”
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This review is one of the preliminary studies on the gynogenesis induction for the production of all female Tiger prawn,Penaeus monodonproject. The author would like to acknowledge all writers who involved for the ideas and reviews contributed along this publication process.
推荐访问:gynogenesis review manipulation
本文来源:http://www.zhangdahai.com/shiyongfanwen/qitafanwen/2023/0428/590541.html