【www.zhangdahai.com--其他范文】
Ming-Liang Feng,Can Wu,Hui-Jing Zhang,Huan Zhou,Tai-Wei Jiao,Meng-Yuan Liu,Ming-Jun Sun
Abstract BACKGROUND The androgen responsive gene,ELL-associated factor 2 (EAF2),expressed in benign prostate tissues,has been shown to play an important role in tumor suppression in a variety of malignant tumors.In addition,some scholars found that EAF2 frameshift mutations are associated with intratumor heterogeneity in colorectal cancer (CRC) and inactivation of EAF2 in microsatellite instability-high CRC.However,the molecular mechanism by which EAF2 is involved in CRC invasion and metastasis remains unclear.AIM To determine the clinical value of expression of EAF2 protein in CRC,and to study the effects of EAF2 on the invasion,migration,and angiogenesis of CRC cells in vitro.METHODS In this study,we collected colorectal adenocarcinoma and corresponding adjacent tissues to investigate the clinical expression of EAF2 protein in patients with advanced CRC.Subsequently,we investigated the effect of EAF2 on the invasion,migration,and angiogenesis of CRC cells in vitro using plasmid transfection.RESULTS EAF2 protein was lowly expressed in cancer tissues of patients with advanced CRC.Kaplan-Meier survival analysis showed that the survival rate of the high EAF2 level group was higher than that of the low EAF2 level group.CONCLUSION Our results demonstrated that EAF2,as a tumor suppressor,may inhibit the invasion,metastasis,and angiogenesis of CRC cells by regulating the signal transducer and activator of transcription 3/transforming growth factor-β1 crosstalk pathway,and play a cancer suppressive and protective role in the occurrence and development of CRC.Our findings are of great significance to provide a new idea and theoretical basis for the targeted diagnosis and treatment of CRC.
Key Words:ELL-associated factor 2;Transforming growth factor-β1;Signal transducer and activator of transcription 3;Colorectal cancer;Invasion;Migration;Angiogenesis
Colorectal cancer (CRC) is one of the tumors with high morbidity and mortality in the world.The Global Cancer Statistics 2020 revealed that CRC was estimated to be the third most diagnosed malignant tumor (10%) and the second leading cause of cancer death (9.4%) worldwide[1].As an important process in the development of CRC,tumor metastasis is a key factor affecting the survival rate of CRC patients.Approximately 35% of newly diagnosed CRC patients present metastatic disease,and about 50% of patients with non-metastatic disease eventually develop metastatic disease[2].Most regrettably,the 5-year survival rate of patients with metastatic CRC is less than 10%[3].There is a great need to elucidate the molecular mechanism of CRC invasion and metastasis,which is beneficial to explore new potential molecular markers and the direction of targeted therapy of CRC.
ELL-associated factor 2 (EAF2) was first identified as an androgen-responsive gene expressed by luminal epithelial cells in benign prostatic tissue[4,5].Interestingly,EAF2 has been found to inhibit cell proliferation and tumor size in prostate cancerin vivo[6],and corresponding to that,knockdown of EAF2 in prostate cancer cells resulted in increased proliferation and migration[7].In recent years,more and more studies have shown that EAF2 plays an important role in tumor suppression in a variety of malignant tumors[8].Furthermore,scholars have identified intratumor heterogeneity (ITH) of EAF2 frameshift mutations in CRC and the inactivation of EAF2 in microsatellite instability (MSI)-high CRC[9].This finding suggests that EAF2 inactivation may occur during tumor progression rather than tumorigenesis.Besides,EAF2 gene silencing could modulate the cytotoxic response of colon cancer cell line HCT116 to statins[10].However,studies on the expression and role of EAF2 protein in CRC are still lacking.The molecular mechanism of involvement of EAF2 in CRC invasion and metastasis remains unclear.
EAF2 has been shown to attenuate transforming growth factor beta 1 (TGF-β1)-induced G1 cell cycle arrest and cell migration in a variety of tumor cells,such as renal carcinoma cells,human hepatocellular carcinoma cells,and breast cancer cells[11].It is worth noting that blocking the TGF-β1 signaling pathway may be a therapeutic strategy for attenuating tumor metastasis in CRC metastatic models[12].In addition,the signal transducer and activator of transcription 3 (STAT3) pathway is involved in the process of TGF-β1-induced epithelial-mesenchymal transition (EMT),and cell migration and invasion in malignant tumor[13,14].As well,EAF2 knockout induced STAT3 phosphorylation (Tyr705) in prostate cancer cells,which drove tumor progression in prostate cancer[15].Therefore,in this study,we investigated the effect of EAF2 on the STAT3/TGF-β1 pathway in CRC cells to explore the possible mechanism of action of EAF2 in CRC.
All tissues in the body need blood vessels to provide nutrients and oxygen to maintain growth and function[16],so angiogenesis provides necessary functions in normal growth and biological development processes,such as embryonic development,bone formation,and the function of ovaries and other endocrine glands[17].Not only that,tumor growth and metastasis also require angiogenesis[18].Abnormalities in vasculogenesis and spermatogenesis have been found in EAF2 knockout mice[19].Besides,EAF2 negatively regulated the activity of angiogenic factor hypoxia inducible factor (HIF)-1α[20].Therefore,exploring the regulatory role of EAF2 in CRC angiogenesis may provide a theoretical basis for anti-angiogenic treatment.Activation of the JAK2/STAT3 (Tyr705) signaling pathway in CRC cells promoted cell proliferation and angiogenesis by positively regulating HIF-1α/vascular endothelial growth factor A (VEGFA)[21,22].Meanwhile,HIF-1α sumoylation may elevate the microvascular density and lumen size and promote tumor growth by amplifying TGF-β/Smad signaling in tumor cells[23,24].Therefore,we further investigated the role of EAF2 in CRC angiogenesisviathe STAT3/TGF-β1 pathway.
In this study,we aimed to determine the clinical value of expression of EAF2 protein in CRC.Furthermore,we studied the effects of EAF2 on the invasion,migration,and angiogenesis of CRC cellsin vitro.In addition,we preliminarily explored the mechanism by which EAF2 affects the biological function and angiogenesis of CRC cellsviaregulating the STAT3/TGF-β1 pathway.Our findings are of great significance to provide a new idea and theoretical basis for the targeted diagnosis and therapy of CRC.
CRC patients and tissue samples
We selected 70 cases of advanced CRC from the First Affiliated Hospital of China Medical University (Liaoning,China) between 2012 and 2015.This study was approved by the Institutional Review Board of the First Affiliated Hospital of China Medical University (Registration No.2021-68-2;informed consent was waived).Seventy pairs of histological sections of colorectal adenocarcinomas and corresponding paracancerous tissue that had been surgically removed were studied.In addition,another eight pairs of fresh cancer and adjacent tissue were selected for western blot assay.None of patients received radiotherapy or chemotherapy prior to surgical resection.We defined tumor stages according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system (8thEdition).
Reagents
Recombinant human TGF-β1 (mammalian derived) was bought from PeproTech (100-21) (United States).The antibodies used in this study include: Rabbit monoclonal anti-EAF2 (ab237753) (for IHC-P),rabbit monoclonal anti-EAF2 [EPR7117(2)] (ab151692) (for western blot) (Abcam,United Kingdom);rabbit monoclonal anti-TGF-β1 (56E4) (#3709),rabbit monoclonal anti-phospho-STAT3 (Tyr705) (#9145),rabbit monoclonal anti-STAT3 (#30835) (Cell Signaling,Danvers,MA,United States);rabbit polyclonal anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (FL-335) (Santa Cruz,CA,United States).
Cell culture
CRC cell lines (SW480,RKO,HCT116,HT29,and HIEC) and the human normal colon cell line NCM460 were purchased from the Cell Bank of the Chinese Academy of Science (Shanghai,China).And these cells were cultured in RPMI-1640 medium (Gibco,Grand Island,NY,United States) containing 10% fetal bovine serum (FBS) (Gibco) at 37 °C with 5% CO2in a humidified incubator.Human umbilical vein endothelial cells (HUVECs) were also purchased from the Cell Bank of the Chinese Academy of Science (Shanghai,China) and cultured in DMEM medium (Gibco) containing 10% FBS at 37 °C with 5% CO2in a humidified incubator.
Cell transfection
The plasmid for overexpressing human EAF2 (GenBank No.NM_018456) was designed and synthesized by GeneChem (Shanghai,China).And three plasmid targets for silencing human STAT3 (GenBank No.NM_139276) were also provided by GeneChem.The sequences are listed in Table 1.The cell transfection was performed using the Lipofectamine 2000 Kit (Invitrogen,Carlsbad,CA,United States).Cells were plated into six-well plates and transfected with 4.0 μg plasmid DNA.Six hours after transfection,cells were treated with medium containing 10% FBS.

Table 1 Gene sequences used for transfection
Immunohistochemistry
Typical CRC tissues and corresponding paracancer tissues were selected.The sections were dewaxed,rehydrated,and heated using a pressure-cooker for antigen retrieval.And then antigenic repair was performed with citric acid.Subsequently,the sections were incubated overnight at 4 °C with rabbit monoclonal anti-EAF2 antibody (1:200 dilution,ab237753),followed by incubation with the secondary antibody for 40 min at 37 °C.Finally,the slides were stained with diaminobenzidine,counter-stained with hematoxylin,air-dried,dehydrated,and mounted.
Double blind reading was performed by two experienced pathologists.We calculated the final staining score by multiplying the positive expression score by the intensity score.Five high-power fields (magnification at 200 ×) were observed on each section.We divided the immunostaining intensity into four categories: 0 (negative immunostaining),1 (weak immunostaining),2 (moderate immunostaining),and 3 (strong immunostaining).And the proportion of positive cells scored from 0 to 4 as follows: (1) 0 (positive cells < 5%);(2) 1 (positive cells 5% to 25%);(3) 2 (positive cells 26% to 50%);(4) 3 (positive cells 51% to 75%);and (5) 4 (positive cells > 75%).
Western blot assay
The tissues and cells were lysed in RIPA lysis buffer containing protease inhibitors such as phenylmethylsulfonyl fluoride.Protein concentrations were measured with a BCA kit (Beyotime Institute of Biotechnology,China).Equal amounts of protein (50 μg per sample) were separated by 10% sodium dodecyl sulfate/polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes at a 200 mA constant current.Primary antibodies for EAF2 (ab151692),TGF-β1,phospho-STAT3 (Tyr705),STAT3,and GAPDH were used at a dilution ratio of 1:1000.Then,anti-rabbit immunoglobulin G secondary antibody (1:5000) was used to incubate the membranes at 37 °C for 2 h.The immunoreactive bands were detected with ECL-Plus chemiluminescent detection reagents (Beyotime Institute of Biotechnology) and using the Microchemi 4.2 Bio-imaging system.The expression of proteins of interest was normalized to that of GAPDH.Experiments were repeated at least three times under the same experimental conditions.
Transwell invasion and migration assays
Before invasion assay,Matrigel (BD Biosciences,San Diego,CA,United States) diluted at a ratio of 1:8 with serum-free medium was used to block the membranes at 37 °C for 6 h.A total of 4 × 104cells/well in 200 μL serum-free medium were seeded in the upper chambers of 8-μM pore transwell plates (3422;Corning Incorporated,NY,United States),and 600 μL medium containing 20% FBS was added to the lower chambers.For migration assay,2 × 105cells/well were seeded into the upper chambers of 8-μM pore transwell plates (3422) in 200 μL serum-free medium.The lower chambers were filled with 600 μL medium with 10% FBS.
After incubation for 72h,the non-invaded or non-migrated cells on the inner surface of the filter membrane were removed by scrubbing with a cotton swab,and the invaded or migrated cells were fixed by methanol.Next,the cells were stained with 0.1% crystal violet.The membranes were moved from the chambers and fixed on cover slides.Invading or metastasizing cells were counted from five random fields under a light microscope (magnification at 200 ×).The experiments were repeated at least three times under the same experimental conditions.
Wound healing assay
A total of 2 × 105cells/well were seeded into 6-well plates.Scratch wounds were generated using a 200 μL pipette tip when the cells reached 90% confluence.Afterwards,the cells were washed with phosphate buffered saline (PBS) three times,and then incubated in medium containing 2% serum.The scratch wounds were photographed at 0 h,6 h,12 h,24 h,48 h,and 72 h in five selected regions.ImageJ software (Version 1.46r) was used to measure the wound area (magnification at 40 ×).The experiments were repeated at least three times under the same experimental conditions.
Tube formation assay
Before tube formation assay,Matrigel was placed in a 96-well plate at 37 °C for 1 h.And the conditioned medium from treated RKO cells was collected and centrifuged at 2000 rpm for 10 min to remove debris.HUVECs at 1.0 × 104cells/well were seeded in a Matrigel coated 96-well plate and cultured in conditioned medium.After 6 h,capillary morphogenesis was evaluated by inverted microscopy (magnification at 100 ×).Then,we analyzed the images for evaluating the branch points by means of the angiogenesis analyzer that was developed for the Image J software (Version 1.46r).
HUVEC transwell migration assay
For HUVEC migration assay,1 × 105HUVECs were plated in the upper chambers of 8-μM pore transwell plates (3422) with 200 μL serum-free medium;600 μL conditioned medium was placed in the lower chambers.After 6 h,the cells were fixed with methanol and non-migrated cells were removed from the inner surface of the filter membrane.The cells on the lower surface of the membrane were stained with 0.1% crystal violet,and the number of migrated cells was counted from five fields (magnification at 200 ×) under an optical microscope.The experiments were repeated at least three times under the same experimental conditions.
HUVEC scratch assay
For scratch assay,2 × 105HUVECs/well were seeded in 6-well plate.When cells reached about 90% confluence,a 200 μL tip was used to scratch the cells.The cells were then washed with PBS three times and incubated with 2 mL conditioned medium per well.The scratch was photographed at 0 h,12 h,and 24 h time points under a microscope in five selected regions (magnification at 40 ×).ImageJ software (Version 1.46r) was used to measure the wound area.Experiments were repeated at least three times under the same experimental conditions.
Statistical analysis
The results in this study were analyzed with SPSS software (Version 26).Pearson’s chi-squared test was adopted to evaluate the correlation between EAF2 protein and clinicopathological parameters.The expression differences of EAF2 between paired adenocarcinoma and paracancerous tissues were analyzed by Wilcoxon sign rank test.Receiver operating characteristic (ROC) curve was used to determine the Youden index to delimit the high and low expression of EAF2 in tissues.The data are shown as the mean ± SD.Student’sttest was used for analyzing the differences between two groups,while for differences among three or more groups,one-way ANOVA was performed.The Kaplan-Meier method was used to estimate the cumulative survival of patients,and the Log-rank test was used to test the significance of the differences in survival.Cox proportional hazards regression model was used for multivariate analysis to evaluate the independent prognostic effect of EAF2 protein on survival.All the statistics were subjected to ANOVA followed by Bonferroni test.Two-sidedPvalues < 0.05 were considered statistically significant.
EAF2 protein expression is decreased in advanced CRC tissue
To assess the expression and localization of EAF2 protein in CRC specimens,we examined the expression of EAF2 at the protein level in paired adenocarcinoma and paracancerous tissues by immunohistochemical analysis and western blot assay.Immunohistochemical analysis showed that EAF2 protein was mainly localized in the cytoplasm of colorectal epithelial cells (Figure 1A).The median EAF2 score was 6.0 in tumor tissue and 6.8 in adjacent non-tumor tissue,with a median difference of -2.0.Moreover,Wilcoxon signed-rank test of paired samples showed that EAF2 expression was lower in tumor tissue than in non-tumor tissue (Z=-3.727,P< 0.001).Additionally,we used western blot assay to measure the level of EAF2 protein expression in eight matched pairs of fresh colorectal adenocarcinoma and corresponding paracancerous tissue.The results also revealed a lower level of EAF2 protein expression (P=0.012) in tumor tissue compared with adjacent non-tumor tissue (Figure 1B).According to the Youden index determined by ROC curve,the cut-off value delimiting high and low expression of EAF2 (dependent variable is non-cancer) was determined to be 6.2 (P< 0.001).In this study,we found low EAF2 expression in 58 of 70 CRC tissues.
We then assessed the relationship between EAF2 protein expression and major clinicopathological characteristics.Statistical results showed that the expression of EAF2 protein in CRC tissue was negatively correlated with distant metastasis (r=-0.268,P=0.025) and carcinoembryonic antigen (CEA) (r=-0.249,P=0.038),but not with other clinical characteristics,such as age,sex,primary tumor site,tumor size,tumor histological differentiation,degree of differentiation,vasculolymphatic and/or perineural invasion,tumor stage,tumor invasion depth,lymph node status,carbohydrate antigen 19-9 (CA19-9),P53,or CDX2 (Table 2).High expression of CEA,CA19-9,P53,and CDX2 was defined as a score of ≥ 3,and their low expression was defined as a score of < 3.
Survival analysis of EAF2 expression in patients with advanced CRC
Kaplan-Meier survival analysis and Log-rank testing for overall survival (OS) showed that the survival rate of the group with high EAF2 level was higher than that of the group with low EAF2 level (P=0.026).Figure 2 shows the Kaplan-Meier survival curves of advanced CRC patients with high and low EAF2 expression.We used Cox proportional-hazards model to evaluate the effect of EAF2 protein on OS in patients with advanced CRC.From univariate analysis,we found that angiolymphatic and/or perineural invasion (P=0.003),tumor stage (P=0.012),tumor invasion depth (P=0.011),lymph node status (P=0.017),distant metastasis (P< 0.001),and EAF2 protein expression level (P=0.038) were significantly correlated with OS (Table 3).The clinicopathological characteristics withP< 0.3 in univariate analysis were included in multivariate analysis,and the independent prognostic effect of EAF2 protein on OS was assessed by adjusting for confounding factors.However,multivariate analysis proved that tumor invasion depth (P=0.035) and distal metastasis (P< 0.001) were independent prognostic factors for OS (Table 3).

Figure 1 ELL-associated factor 2 protein is decreased in colorectal cancer tissue. A: Representative immunohistochemical staining for ELLassociated factor 2 (EAF2) in colorectal adenocarcinoma tissue and adjacent tissue (magnification,200 × and 400 ×);B: western blot showing lower EAF2 level in colorectal adenocarcinoma fresh tissue than in corresponding para-cancerous tissue (T,colorectal adenocarcinoma tissue;N,adjacent non-tumor tissue).EAF2: ELL-associated factor 2;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.

Figure 2 Kaplan-Meier survival curves for ELL-associated factor 2 expression in colorectal cancer tissue.The survival rate of the group with high ELL-associated factor 2 (EAF2) levels was higher than that of the group with low EAF2 levels.EAF2: ELL-associated factor 2.
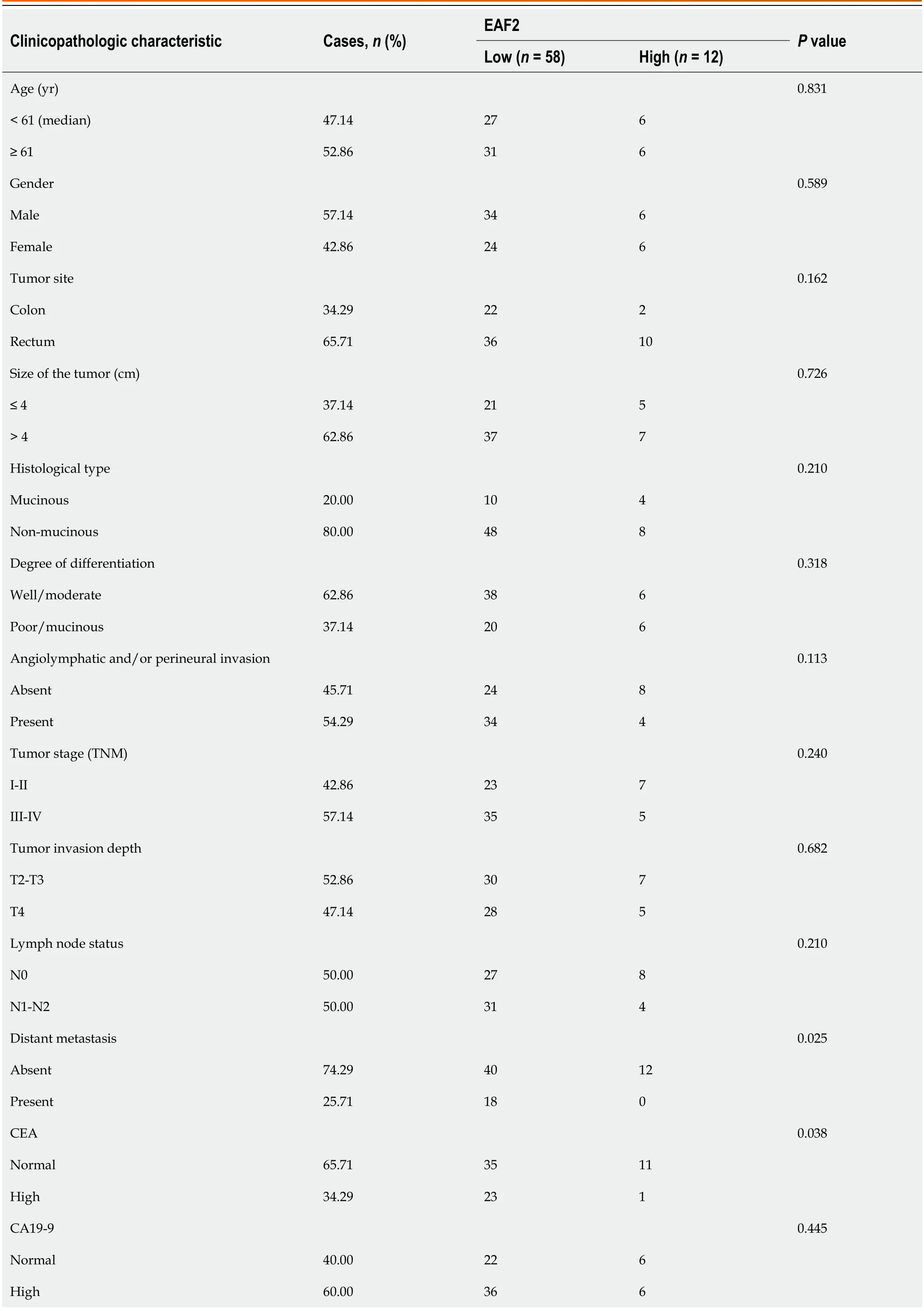
Table 2 Baseline characteristics of patients with advanced colorectal cancer and their correlation with immunohistochemical expression of ELL-associated factor 2

EAF2: ELL-associated factor 2;TNM: Tumor-node-metastasis;CEA: Carcinoembryonic antigen;CA19-9: Carbohydrate antigen 19-9.

Table 3 Univariate and multivariate survival analyses of overall survival for colorectal cancer by Cox regression
Overexpression of EAF2 inhibits invasion and migration of CRC cells
We cultured CRC cellsin vitroto determine the expression of EAF2 protein and further investigate its effects on the biological function of CRC cells.The expression level of EAF2 protein in human CRC cell lines (SW480,RKO,HCT116,HT29,and HIEC) were observably lower than that in normal colorectal epithelial cells (NCM460) (Figure 3).There was a considerable decrease (64.11%) in EAF2 protein expression in colon cancer RKO cells (P< 0.001) (Figure 3).Thus,we used RKO cells in subsequent experiments.
Subsequently,we overexpressed EAF2 protein by plasmid transfection technique to investigate the effects of EAF2 overexpression on the invasion and migration of RKO cells.EAF2 overexpression significantly increased the protein expression level in RKO cells,which was 2.25-fold higher than that of the control group (Figure 4A,P< 0.001).Results of transwell invasion assay showed that EAF2 overexpression significantly weakened the invasion ability of RKO cells (Figure 4B,P< 0.001).Analogously,cells transfected with EAF2-overexpressing plasmid also showed a poorer migration capacity than the control group in the transwell migration assay (Figure 4B,P< 0.001).In addition,as shown in Figure 4C,EAF2 overexpression reduced the wound healing ability of RKO cells (P< 0.001).
Overexpression of EAF2 suppresses the activity of STAT3/TGF-β1 crosstalk pathway
In this study,we set out to investigate the molecular mechanism of EAF2 on RKO cellsin vitro.Phosphorylated STAT3 (Tyr705) and TGF-β1 protein expression levels were significantly increased in human CRC cell lines (SW480,RKO,HCT116,HT29,and HIEC) compared with normal colorectal epithelial cells (NCM460) (Figure 3).The upregulation of phosphorylated STAT3 protein (Tyr705) (with a 1.585-fold increase,P< 0.001) and TGF-β1 protein (with a 2.485-fold increase,P< 0.001) in RKO cells was statistically significant.
Compared with control cells,TGF-β1 protein expression level was significantly decreased in RKO cells transfected with EAF2-overexpressing plasmid (Figure 5A).Meanwhile,we also found that overexpression of EAF2 remarkably decreased phosphorylated STAT3 (Tyr705) levels in RKO cells,but not total STAT3 levels (Figure 5A).
And then we knocked down STAT3 gene by transfecting with STAT3 siRNA (Figure 6A).We found that inhibition of STAT3 phosphorylation (with a reduction of 60.25%,P< 0.001) resulted in a significant decrease in TGF-β1 protein expression (P< 0.001) in RKO cells but not in EAF2 (P=0.228) (Figure 6B).Furthermore,we treated RKO cells with TGF-β1 recombinant protein to clarify the regulatory effect of TGF-β1 on EAF2 protein expression and STAT3 phosphorylation.The results revealed that TGF-β1 recombinant protein reversed the decrease of phosphorylated STAT3 (Tyr705) induced by EAF2 overexpression (P< 0.001) (Figure 5A).However,our results showed that TGF-β1 recombinant protein did not affect the expression of EAF2 protein (P=0.099) in RKO cells (Figure 5A).

Figure 3 Protein levels of ELL-associated factor 2,phosphorylated-signal transducer and activator of transcription 3,and transforming growth factor-β1 in colorectal cancer cell lines (SW480,RKO,HCT116,HT29,and HIEC) and normal colorectal epithelial cells (NCM460)by western blot assay.A: Western blot results of proteins in in colorectal cancer cell lines (SW480,RKO,HCT116,HT29,and HIEC) and normal colorectal epithelial cells (NCM460);B: Relative expression of the proteins.The data are shown as the mean ± SD.aP < 0.05,bP < 0.001.p-STAT3: Phosphorylated-signal transducer and activator of transcription 3;TGF-β1: Transforming growth factor-β1;EAF2: ELL-associated factor 2;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Overexpression of EAF2 inhibits invasion and migration of CRC cells by down-regulating the activity of STAT3/TGF-β1 crosstalk pathway
We attempted to further determine whether EAF2 regulates the biological function of CRC cells by regulating the activity of STAT3/TGF-β1 crosstalk pathway.We used TGF-β1 recombinant protein to treat the RKO cells transfected with EAF2-overexpressing plasmid for 24 h.As a result,the invasiveness and metastasis capability of RKO cells weakened by EAF2 overexpression was partially reversed by TGF-β1 recombinant protein (Figures 5B and 5C).
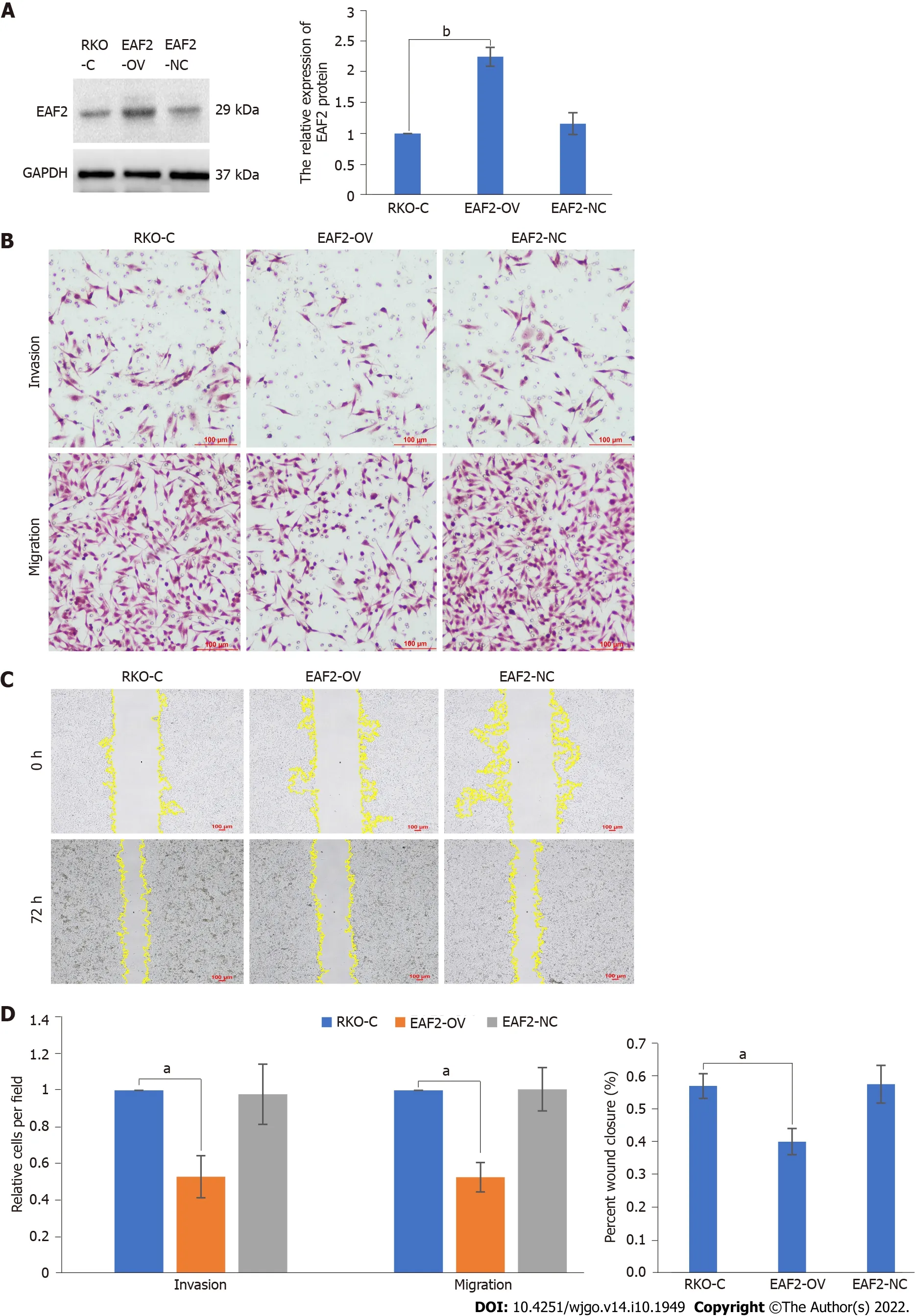
Figure 4 Overexpression of ELL-associated factor 2 inhibits the invasion and migration of RKO cells. A: Protein levels of ELL-associated factor 2 (EAF2) in RKO cells transfected with EAF2 overexpression vector by western blot assay;B: Invasion and migration abilities evaluated by transwell assay;C: Migration ability analyzed by wound healing assay after 72 h;D: Migration and invasion abilities of RKO cells measured using ImageJ software.The data are shown as the mean ± SD.aP < 0.001;bP < 0.05.RKO-C: The group of RKO control cells;EAF2-OV: The group of RKO cells transfected with ELL-associated factor 2 overexpression vector;EAF2-NC: The group of RKO cells transfected empty vector;EAF2: ELL-associated factor 2;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
Overexpression of EAF2 inhibits CRC angiogenesis
In this study,we preliminarily investigated the effects of RKO cells overexpressing EAF2 on the migration and tube formation abilities of HUVECs.First,HUVECs were cultured with DMEM medium containing 10% serum[25].After 2-6 passages of cells,HUVECs were subcultured into corresponding petri dishes.We then treated HUVECs with conditioned medium from RKO cells transfected with EAF2-overexpressed plasmid (EAF2-OV group) or empty control plasmid (EAF2-NC group) for 24 h.Compared with the control group (RKO-C group),whose conditioned medium was collected from RKO cells cultured with 10% serum for 24 h,the tube formation (Figure 7A) and migration (Figures 7B and 7C) of HUVECs in the EAF2-OV group were significantly reduced,while the differences in the EAF2-NC group were not statistically significant.Our results indicated that overexpression of EAF2 protein may inhibit angiogenesis induced by CRC cellsin vitro.
Overexpression of EAF2 regulates CRC angiogenesis via STAT3/TGF-β1 signaling pathway
STAT3-related pathway and non-specific angiogenic factor TGF-β1 play a proangiogenic role in tumor angiogenesis.Our study showed that overexpression of EAF2 inhibits the activity of the STAT3/TGF-β1 crosstalk pathway in RKO cells.Hence,we next investigated whether EAF2 inhibits CRC angiogenesis through the STAT3/TGF-β1 signaling pathway.We used TGF-β1 recombinant protein to treat RKO cells transfected with or without EAF2-overexpressing plasmid for 24 h,and collected conditioned medium to culture HUVECs.We aimed to investigate whether TGF-β1-related pathways are involved in the molecular mechanism of EAF2 overexpression regulating angiogenesis in RKO cellsin vitro.Compared with the RKO-C group,the tube formation (Figure 8A) and migration (Figures 8B and 8C) of HUVECs in the EAF2-OV group were significantly decreased.Interestingly,when HUVECs were cultured with conditioned medium from EAF2-overexpressing RKO cells treated with TGF-β1 recombinant protein,the inhibition of EAF2 overexpression on tube formation (Figure 8A) and migration (Figures 8B and 8C) of HUVECs was reversed.Together,these data confirmed our hypothesis that EAF2 overexpression inhibits CRC angiogenesis by suppressing the activity of the STAT3/TGF-β1 crosstalk pathwayin vitro.

Figure 5 Overexpression of ELL-associated factor 2 suppresses the activity of the signal transducer and activator of transcription 3/transforming growth factor-β1 pathway to inhibit the invasion and migration of RKO cells. A: Overexpression of ELL-associated factor 2 (EAF2) inhibited the signal transducer and activator of transcription 3/transforming growth factor-β1 (STAT3/TGF-β1) pathway measured by western blot assay.And TGF-β1 recombinant protein abolished EAF2 overexpression-reduced the phosphorylation (Tyr705) of STAT3 protein;B: Invasion and migration abilities evaluated by transwelll assay;C: Migration ability analyzed by wound healing assay after 72 h;D: Migration and invasion abilities of RKO cells measured using ImageJ software.The data are shown as the mean ± SD.aP < 0.05,bP < 0.001.RKO-C: The group of RKO control cells;EAF2-OV: The group of RKO cells transfected with ELLassociated factor 2 overexpression vector;RKO+T: The group of RKO cells treated with 5 ng/mL transforming growth factor-β1 recombinant protein for 24 h;EAF2-OV+T: The group of RKO cells were transfected with EAF2-OV plasmid for 24 h and then cultured with 5 ng/mL transforming growth factor-β1 recombinant protein for 24 h;p-STAT3: Phosphorylated-signal transducer and activator of transcription 3;TGF-β1: Transforming growth factor-β1;EAF2: ELL-associated factor 2;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.

Figure 6 Silencing signal transducer and activator of transcription 3 decreases transforming growth factor-β1 protein expression in RKO cells. A: Protein levels of phosphorylated-signal transducer and activator of transcription 3 (p-STAT3) in RKO cells transfected with STAT3 siRNA plasmid by western blot assay;B: Knockdown of STAT3 decreased the levels of transforming growth factor-β1 protein but not ELL-associated factor 2 protein in RKO cells.The data are shown as the mean ± SD.aP < 0.01.RKO-C: The group of RKO control cells;STAT3-si: The group of RKO cells transfected with signal transducer and activator of transcription 3 siRNA plasmid.STAT3-NC: The group of RKO cells transfected empty vector;p-STAT3: Phosphorylated-signal transducer and activator of transcription 3;TGF-β1: Transforming growth factor-β1;EAF2: ELL-associated factor 2;GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.

Figure 7 Overexpression of ELL-associated factor 2 in RKO cells inhibits tumor-induced angiogenesis.A: Conditioned medium from RKO cells transfected with ELL-associated factor 2-overexpressed plasmids or empty control plasmids was collected to perform tube formation assay;B: Migration ability of human umbilical vein endothelial cells (HUVECs) analyzed by transwell migration assay;C: Migration ability of HUVECs analyzed by wound healing assay.And the results were measured with ImageJ software.The data are shown as the mean ± SD.aP < 0.001.HUVECs: Human Umbilical Vein Endothelial Cells;RKO-C: The group of Human Umbilical Vein Endothelial Cells cultured with conditioned medium from RKO cells cultured with 10% serum for 24 h;EAF2-OV: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells transfected with ELL-associated factor 2-overexpressed plasmids for 24 h;EAF2-NC: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells transfected with empty control plasmids for 24 h.

Figure 8 Overexpression of ELL-associated factor 2 inhibited tumor-induced angiogenesis in RKO cells via the signal transducer and activator of transcription 3/transforming growth factor beta 1 pathway.A: Conditioned medium from RKO cells transfected with ELL-associated factor 2-overexpressed plasmids or treated with transforming growth factor beta 1 recombinant protein was collected to perform tube formation assay;B: Migration ability of human umbilical vein endothelial cells (HUVECs) analyzed by transwell migration assay;C: Migration ability of HUVECs analyzed by wound healing assay.And the results were measured with ImageJ software.The data are shown as the mean ± SD.aP < 0.05,bP < 0.001.RKO-C: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells cultured with 10% serum for 24 h;EAF2-OV: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells transfected with ELL-associated factor 2-overexpressed plasmids for 24 h;RKO+T: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells treated with 5 ng/mL transforming growth factor-β1 pathway recombinant protein for 24 h;EAF2-OV+T: The group of human umbilical vein endothelial cells cultured with conditioned medium from RKO cells which were transfected with EAF-OV plasmid for 24 h and then cultured with 5 ng/mL transforming growth factor beta 1 pathway recombinant protein for 24 h.

Figure 9 Schematic depiction of ELL-associated factor 2 regulating the signal transducer and activator of transcription 3/transforming growth factor beta 1 crosstalk pathway in this study. EAF2: ELL-associated factor 2;p-STAT3: Phosphorylated-signal transducer and activator of transcription 3;TGF-β1: Transforming growth factor beta 1;CRC: Colorectal cancer;HUVECs: Human umbilical vein endothelial cells.
Previous studies have supported the role of EAF2 as a tumor suppressor in multiple human tissues[6].EAF2 knockout mice developed a higher frequency of B-cell lymphoma,lung adenocarcinoma,hepatocellular carcinoma,and prostatic intraepithelial neoplasia[6].EAF2 and its homologue EAF1 were originally identified as binding partners of the fusion protein ELL (11-19 lysine-rich leukemia) associated with myeloid leukemia[26,27],stimulating the extension activity of ELL.However,EAF2 mutations produce truncated EAF2 mutants,which may be associated with the pathogenesis of MSIhigh cancers[9].Recently,further studies have found ITH in EAF2 frameshift mutations in CRC and the inactivation of EAF2 in MSI-high CRC[9],suggesting that EAF2 mutations may occur during tumor progression rather than during tumorigenesis.However,the expression,role,and molecular mechanism of EAF2 protein in CRC remain unclear.
This study collected colorectal adenocarcinoma and paired adjacent tissues to investigate the clinical expression of EAF2 protein in patients with advanced CRC.Compared with non-tumor tissue,the results of immunohistochemistry and western blot assay showed that the level of EAF2 protein in CRC tissue was significantly lower.And immunohistochemical results indicated that EAF2 protein was mainly expressed in the cytoplasm.Meanwhile,in vitroexperiments also confirmed that EAF2 expression level in CRC cells was lower than that in normal colorectal epithelial cells.Further analysis of the correlation between EAF2 expression level and clinicopathological characteristics revealed that EAF2 protein level was negatively correlated with distant metastasis and CEA expression level in CRC tissues.These results suggest that EAF2 protein may be related to the invasion and metastasis of CRC.However,further studies are needed to determine the mechanism of decreased EAF2 protein expression in CRC.
Besides,Kaplan-Meier survival analysis showed that the survival rate of the group with high EAF2 levels was higher than that of the group with low EAF2 levels.Although multivariate analysis did not suggest that EAF2 expression level was an independent survival prognostic marker in patients with advanced CRC,univariate analysis suggested that it was an influential factor of survival and prognosis in patients with advanced CRC.However,more cases and more comprehensive clinical studies are needed to further explore this question.
In addition,phospho-STAT3 (Tyr705) and TGF-β1 protein were highly expressed in CRC cells in this study.Studies have shown that TGF-β1 is highly expressed in CRC tissue,and overexpression of TGF-β1 facilitates the invasion and migration of CRC cellsin vitro[28].Furthermore,blocking the TGF-β1 signaling pathway in CRC metastatic models may serve as a therapeutic strategy to weaken tumor metastasisin vivo[20].Importantly,STAT3 acts as a positive regulator to activate TGF-β1 to induce tumor EMT and metastasis[14].Meanwhile,STAT3 is one of the major oncogenic pathways activated in CRC,and its activation can be detected in CRC tissue,primary CRC cells,or CRC cell lines[29-31].
Further studies have revealed that EAF2 attenuates TGF-β1-induced G1 cell cycle arrest and cell migration,which has been demonstrated in renal carcinoma cells,human hepatocellular carcinoma cells,and breast cancer cells[11].Besides,phospho-STAT3 (Tyr705) immunostaining is correlated with down-regulation of EAF2 in human prostate cancer specimens[15].EAF2 knockout induces STAT3 phosphorylation (Tyr705)in vivoandin vitro,suggesting that EAF2 is a repressor of the STAT3 signaling pathway[15].In our study,we found that overexpression of EAF2 decreased the levels of phosphorylated STAT3 (Tyr705) and TGF-β1 protein in CRC cells.In addition to this,we also found that silencing STAT3 reduced TGF-β1 protein expression.More than that,TGF-β1 recombinant protein eliminated the reduction of phosphorylated STAT3 (Tyr705) induced by EAF2 overexpression.Together,these results indicate that EAF2 overexpression may inhibit the activity of STAT3/TGF-β1 crosstalk pathway in CRC cells.
We found that EAF2 overexpression restrains the invasion and metastasis of CRC cells.It makes sense to further explore the role of EAF2 in regulating the activity of the STAT3/TGF-β1 crosstalk pathway in CRC cell invasion and metastasis.The results revealed that the invasiveness and metastasis capability of CRC cells weakened by EAF2 overexpression could be partially reversed by TGF-β1 protein.Therefore,it was concluded that EAF2 overexpression inhibited the invasion and migration of CRC cells by down-regulating the activity of the STAT3/TGF-β1 crosstalk pathway.It is possible that therapeutic drugs targeting the STAT3/TGF-β1 signaling pathway may be effective for CRC subsets with down-regulated EAF2,elevated STAT3 phosphorylation,and up-regulated TGF-β1.
Tumor angiogenesis is critical not only for tumor growth,but also for tumor progression and metastasis[32].Tumor angiogenesis is regulated by multiple angiogenic factors and angiogenic inhibitors[21].The activation of the STAT3 pathway contributes to angiogenesis by increasing the expression of angiogenic factors,such as VEGFA,interleukin (IL)-8,HIF-1α,or IL-6[33].The non-specific angiogenic factor TGF-β1 plays a proangiogenic role in CRC angiogenesis[34].TGF-β1 signaling inactivation caused by dnTGFBR1 in tumor cells can reduce microvessel density and lumen sizes,and decrease tumor growth[35].In keloid tissues,sumoylation of HIF-1α may increase the stability and an amplified effect on TGF-β/SMAD signaling[23].Matrigel plug angiogenesis assay showed that EAF2 knockout mice responded to VEGF with significantly enhanced neovascularization[19].And EAF2 is a negative regulator of HIF-1α activity[20].EAF2 binds to and stabilizes von Hippel-Lindau protein and then disrupts the HIF-1α-mediated hypoxia signaling pathway[19,36].Abnormal vasculogenesis occurred in EAF2 knockout mice[19].We hypothesized that EAF2 may regulate CRC angiogenesis through the STAT3/TGF-β1 pathway.HUVECs were used in this study for evaluating the impact of RKO cells on angiogenesis.Besides,endothelial progenitor cells are also a good endothelial model for studying tumor-induced angiogenesis[37].
In this study,our results show that EAF2-overexpressing CRC cells can inhibit tube formation and migration of HUVECs.Besides,the inhibition of EAF2 overexpression on tube formation and migration of HUVECs may be reversed by TGF-β1 recombinant protein.This suggests that EAF2 overexpression inhibited CRC angiogenesis by suppressing the activity of the STAT3/TGF-β1 crosstalk pathway.However,this study still has some limitations,and lacks further studies on vascular regulatory factors released by vascular endothelial cells and changes in tumor microenvironment mediated by EAF2 and its downstream STAT3/TGF-β1 crosstalk pathway.
In conclusion,our findings suggest that EAF2 protein is under-expressed in cancer tissue of patients with advanced CRC.And Kaplan-Meier survival analysis showed that the survival rate of the group with high EAF2 levels was higher than that of the group with low EAF2 levels.Moreover,as a tumor suppressor,EAF2 may play a tumor suppressive and protective role in the development of CRC by inhibiting the invasion,metastasis,and angiogenesis of CRC cells.The regulatory effect of EAF2 on the biological functions of CRC cells may be realized by regulating the STAT3/TGF-β1 crosstalk pathway (Figure 9).Further research on methods and technologies to increase EAF2 in CRC will be one of the future research directions,and exploration of upstream factors such as related non-coding RNAs may provide new diagnostic markers and novel therapeutic targets for CRC.
Research background
There are few studies on the expression and role of ELL-associated factor 2 (EAF2) protein in colorectal cancer (CRC).The molecular mechanism of involvement of EAF2 in CRC invasion and metastasis remains unclear.
Research motivation
EAF2 may play a tumor suppressive and protective role in the development of CRC by inhibiting the invasion,metastasis,and angiogenesis of CRC cells.
Research objectives
We assessed the expression and localization of EAF2 protein in CRC specimens.Meanwhile,we cultured CRC cellsin vitroto determine the expression of EAF2 protein and further investigate its effects on the biological function of CRC cells.
Research methods
We used immunohistochemistry and western blot assay to detect the expression of EAF2 protein in CRC tissues from 70 patients.In addition,we applied plasmid transfection technology to study the effects of EAF2 on the invasion,migration,and angiogenesis of CRC cellsin vitro.
Research results
EAF2 protein was lowly expressed in cancer tissues of patients with advanced CRC.Kaplan-Meier survival analysis showed that the survival rate of the group with high EAF2 level was higher than that of the group with low EAF2 level.In addition,EAF2 affected the biological functions of CRC cells by regulating the STAT3/TGF-β1 crosstalk pathway.
Research conclusions
As a tumor suppressor,EAF2 may play a tumor suppressive and protective role in the development of CRC by inhibiting the invasion,metastasis,and angiogenesis of CRC cellsviaregulating the signal transducer and activator of transcription 3/transforming growth factor beta 1 crosstalk pathway.These findings may provide new diagnostic markers and novel therapeutic targets for CRC.
Research perspectives
Our data is beneficial to elucidate the molecular mechanism of CRC invasion and metastasis as well as explore new potential molecular markers and the direction of targeted therapy.
Author contributions:Feng ML and Wu C designed the research study;Zhang HJ and Jiao TW performed the research;Zhou H and Liu MY contributed new reagents and analytic tools;Feng ML and Sun MJ analyzed the data and wrote the manuscript;and all authors have read and approved the final manuscript.
Supported bythe Natural Science Foundation of Liaoning Province,No.2019-BS-279.
Institutional review board statement:This study was approved by the Institutional Review Board of The First Affiliated Hospital of China Medical University (Registration No.2021-68-2).
Informed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
Data sharing statement:No additional data are available.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Ming-Liang Feng 0000-0001-6624-9624;Can Wu 0000-0002-9532-8767;Hui-Jing Zhang 0000-0002-6322-2335;Huan Zhou 0000-0002-6299-2514;Tai-Wei Jiao 0000-0002-7679-6354;Meng-Yuan Liu 0000-0002-5058-1558;Ming-Jun Sun 0000-0003-0072-2575.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Wang JJ
推荐访问:factor suppresses Overexpression
本文来源:http://www.zhangdahai.com/shiyongfanwen/qitafanwen/2023/0604/607110.html