【www.zhangdahai.com--其他范文】
董倩, 俞坤武, 杜以梅
TRPV4在心血管疾病中作用的研究进展*
董倩, 俞坤武, 杜以梅△
(华中科技大学同济医学院附属协和医院心内科,湖北 武汉 430000)
瞬时受体电位阳离子通道V亚家族成员4;
心血管疾病;
机制
瞬时受体电位阳离子通道V亚家族成员4(transient receptor potential cation channel subfamily V member 4, TRPV4)是瞬时受体电位阳离子通道(transient receptor potential cation channel, TRP)家族成员,是一种非选择性阳离子通道,对Ca2+离子具有适中通透性,对Na+和Mg2+少量通透(Ca/Na约为6~10,Ca/Mg约为2~3)。TRPV4在心血管系统表达广泛,主要表达于血管内皮细胞、血管平滑肌细胞、心肌细胞和成纤维细胞等,可调节血管张力和血管通透性,参与机械信号传导等。已知TRPV4的表达和功能异常,参与了高血压、动脉粥样硬化、心肌缺血再灌注损伤、心力衰竭和肺水肿、心律失常等疾病的发生发展,本文旨在对心血管系统TRPV4的生理功能及其异常导致的相关疾病进行概述。
TRPV4是一种渗透机械敏感性的非选择性阳离子通道,主要对Ca2+离子通透。TRPV4是由具有六个跨膜结构域的亚基形成的对称四聚体,每个亚基共871个氨基酸,包含6次跨膜结构域(S1~S6),N端和C端均位于胞内,S5和S6以及它们之间的P环构成通透阳离子的孔区。此外,TRPV4也可与TRP家族其他单体(如TRPC1和TRPP2)组成异源四聚体发挥功能[1]。TRPV4广泛表达在心血管系统,既可被机械(热、肿胀、剪切力)和化学(花生四烯酸及其代谢产物、内源性大麻素、ATP、钙调蛋白、4α-PDD、GSK1016790A)等多种刺激激活,也可被钌红、链霉素、AB159908、HC-067047、GSK2193874和RN-1734等选择性阻断[2-3]。现已阐明TRPV4在生理状态下维持机体渗透压平衡和内皮细胞屏障,并调节血管通透性[4]。TRPV4激活后介导胞内Ca2+浓度的升高,不仅参与体温调节、渗透压、血管舒张等生理过程,同时参与缺血再灌注损伤、心律失常、心肌肥大、纤维化等病理过程[5-6]。
2.1TRPV4在高血压中的作用高血压发病机制复杂,其中内皮细胞功能障碍与高血压联系密切,两者互为因果。研究表明,TRPV4是内皮细胞(endothelial cell, EC)中Ca2+流入的重要途径[7],主要通过与肌内皮映射区(myoendothelial projection, MEP)定位的蛋白激酶C(protein kinase C, PKC)锚定蛋白150(A-kinase anchoring protein 150, AKAP150)组成耦合门控通道,介导胞外的Ca2+内流从而使得血管舒张[8]。见图1。目前研究显示,高血压的危险因素,如肥胖、高盐饮食、压力应激等均可导致内皮功能障碍,加速高血压的发生发展。Ottolini等[9]报道,高脂饮食可诱导肥胖小鼠血管EC中以一氧化氮(nitric oxide, NO)为底物的过氧亚硝酸盐生成增多,进一步损伤AKAP150EC-TRPV4EC介导的钙离子信号通路,使得血管舒张受阻,导致肥胖小鼠静息状态平均动脉血压升高,从而揭示了肥胖所致高血压的新机制。越来越多的证据表明,TRPV4在调节活性氧(reactive oxygen species, ROS)产生和血管舒张中起着重要作用。NADPH氧化酶2(NADPH oxidase 2, Nox2)已被证明是ROS生成的重要调节器[10]。以往的研究也证明,TRPV4-Nox2能够形成复合物,调节氧化应激和血管舒张反应[11]。国内马鑫教授团队研究显示,肥胖和ROS可导致小鼠血管内皮细胞TPRV4功能受损与血管舒张功能障碍;
相反,通过调整肥胖小鼠的饮食结构或阻断TRPV4与Nox2的耦合,可激活TRPV4的活性,改善血管的舒张功能[12-13],可见,TRPV4功能受损在肥胖症小鼠血管内皮功能紊乱中扮演重要角色。此外,在高盐诱导的高血压小鼠模型中,葛根素可通过激活TRPV4-IKCa/SKCa轴,诱导小鼠肠系膜动脉的内皮依赖性舒张,有效降低血压[14]。最近,马鑫教授团队开发了一种小分子药物JNc-463,它可以增加TRPV4与主动脉内皮型NO合酶(endothelial NO synthase, eNOS)相互作用以增强血管舒张从而在小鼠中发挥抗高血压作用[15]。由此可见,TRPV4可能成为治疗高血压新靶点,尤其适用于合并肥胖等相关危险因素的高血压人群。然而,靶向血管内皮细胞TRPV4的药物研发、进一步的临床试验、甚至药物安全性皆有待研究。
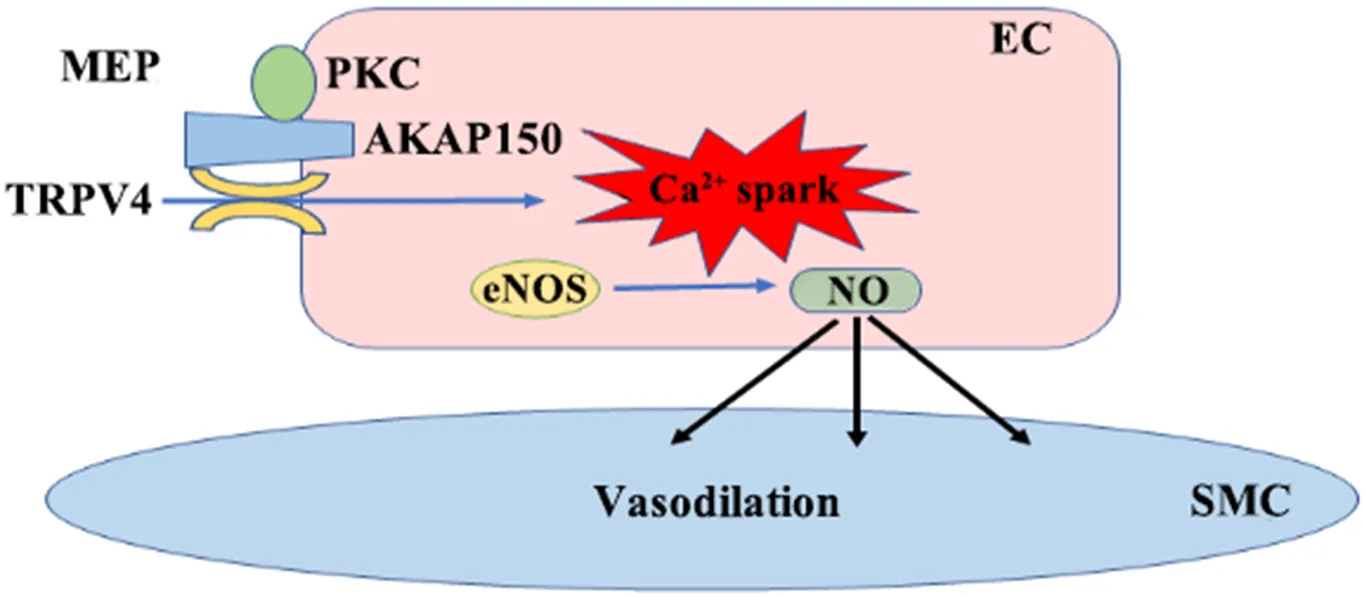
Figure 1. Function of TRPV4 in the vasculature. MEP:
myoendothelial projection. EC:
Endothelialcells. SMC:
Smooth muscle cells;
PKC:
Protein kinase C;
AKAP150:
A-kinase anchoring protein 150;
eNOS:
endothelial NOS;
NO:
nitric oxide.
2.2TRPV4通道在动脉粥样硬化中的作用已知内皮功能障碍、白细胞粘附和巨噬细胞泡沫化是动脉粥样硬化的标志[16]。Xu等研究显示:TRPV4激动剂GSK1016790A可通过CaMKK/AMPK通路促进主动脉内皮细胞中eNOS的磷酸化,并减少TNF-α诱导的单核细胞和内皮细胞粘附,在体实验进一步验证口服GSK1016790A可减少-/-小鼠的动脉粥样硬化斑块形成,表明激活TRPV4可能作为治疗动脉粥样硬化的潜在方法[17]。然而,另一项研究表明,牙龈卟啉单胞菌脂多糖或机械压力刺激可激活TRPV4,加剧巨噬细胞泡沫化,预示阻断或敲除TRPV4可能对动脉粥样硬化有治疗作用,但未进一步行动物实验和机制研究[18]。另外,腹主动脉瘤(abdominal aortic aneurysm, AAA)死亡率极高,而动脉粥样硬化是其重要的病因[19]。Shannon等验证,在弹性蛋白酶处理的WT小鼠和Ang II诱导的-/-小鼠所建立的AAA模型中,阻断TRPV4可抑制中性粒细胞的跨内皮迁移和炎症因子的释放,从而延缓AAA形成[20]。上述研究结果表明TRPV4在动脉粥样硬化中的作用不一致,甚至相反,故TRPV4在动脉粥样硬化中的确切作用有待深入探讨。
2.3TRPV4通道在心肌梗死中的作用急性心肌梗死是全球死亡率最高的疾病之一,急性心肌梗死后进行血运重建是有效的治疗方法,但缺血再灌注后可伴发心肌损伤加重即心肌缺血再灌注损伤(myocardial ischemia/reperfusion injury, MIRI)。目前普遍认为MIRI的发生机制与再灌注后Ca2+超载、ROS攻击、以及炎症反应等有关[21]。TRPV4激活后可升高[Ca2+]i,导致Ca2+超载,从而介导心肌损伤。我们课题组前期通过建立MIRI小鼠模型,观察到MIRI(1~72 h)后,梗死周边心肌组织TRPV4表达明显增多,而腹腔注射TRPV4阻断剂HC-06747可显著缩小MIRI小鼠的心梗面积,而其机制与抑制心肌细胞凋亡、ROS的产生和炎症细胞的浸润有关[22]。我们及他人课题组建立心肌细胞缺氧模型,进一步证实阻断TRPV4通道减轻心肌细胞[Ca2+]i,可通过活化AKT/Nrf2途径,增加抗氧化酶活性,使ROS产生减少,减轻线粒体损伤,从而减少心肌细胞凋亡坏死[23-25]。为了深入探讨TRPV4参与心肌缺血/再灌注(ischemia/reperfusion, I/R)的机制,我们分离小鼠心脏进行Langendorff灌流试验,观察到TRPV4可通过促进JNK-CaMKII磷酸化,从而导致细胞凋亡,加剧心肌I/R损伤。而阻断TRPV4可抑制GSK101诱导的上述作用[26]。此外,在老年小鼠离体灌流I/R模型中也验证了TRPV4的表达和功能显著增强,而阻断TRPV4可抑制老年小鼠MIRI诱导的心肌细胞死亡,该研究对MI风险增加的老年人群具有潜在临床意义[27]。基于目前已有的研究,我们推测在心肌I/R中,TRPV4有望成为治疗MIRI的新靶点。
纤维化主要由肌成纤维细胞产生过多的细胞外基质(extracellular matrix, ECM)引起,是MI后心室重塑的关键机制,而Ca2+信号传导对调控心肌纤维化至关重要。研究表明,TRPV4通道可促进TGF-β1诱导的心肌成纤维细胞分化为肌成纤维细胞,加剧MI后心脏重塑[28]。近期,Adapala团队研究表明,在MI后8周,与WT小鼠相比,TRPV4的缺失保留了小鼠的心脏功能,Masson染色显示梗死区域纤维化显著减轻,更令人意外的是梗死周边区无纤维化,而进一步验证显示敲除可通过Rho/MRTF-A途径抑制心脏成纤维细胞的分化,改善MI后心室重塑[29]。见图2。上述研究表明,TRPV4在心肌缺血后心肌细胞凋亡和心脏纤维化过程中起重要作用。因此,抑制TRPV4可用于防治急性心肌梗死和MIRI。

Figure 2. The mechanisms of TRPV4-induced myocardial ischemia/reperfusion injury and fibrosis. I/R:
ischemia/reperfusion;
JNK-P:
c-Jun N-terminal kinase phosphorylation;
CaMKII-P:
calmodulin-dependent protein kinase II phosphorylation;
ROS:
reactive oxygen species;
mPTP:
mitochondrial permeability transition pore;
MRTF-A:
myocardin-related transcription factor-A.
2.4TRPV4通道在心力衰竭中的作用心力衰竭(heart failure, HF)不仅影响心脏和血管,还影响肺脏。随着肺血管中压力升高,最终引起肺静脉压升高和肺水肿,是心衰患者死亡的主要原因。心衰传统治疗策略侧重于降低肺血管内压力,即通过利尿剂减少总血管内容量,或者通过血管扩张剂减少后负荷。鉴于TRPV4在维持肺血管内皮完整性中的重要作用,以TRPV4为靶点或可改善心衰患者的临床预后,已有研究显示激活TRPV4可使内皮细胞分离,进而破坏肺内皮屏障导致肺水肿和肺泡液的增加[30-31]。见图3。Thorneloe等[32]观察显示:心衰患者肺血管内皮和肺血管平滑肌内TRPV4的表达显著增强,而TRPV4阻断剂GSK2193874(30 nmol/L)可抑制高静脉压(30 cmH2O)灌注引起的肺水肿。在大鼠主动脉缩窄和小鼠心梗所致的心衰模型中,口服GSK2193874可抑制肺静脉的升高,有效缓解肺水肿,增加血氧含量。另外,在化学气体诱导的肺损伤中,内皮损伤所致蛋白的漏出也是肺水肿形成的原因之一,TRPV4阻断剂GSK2220961和GSK2337429还可以降低肺泡灌洗液中蛋白的浓度和炎症细胞的浸润[33]。进一步,TRPV4阻断剂在临床2a期试验中显示出较好的疗效,可有效改善急慢性心衰患者肺充血,这或许将成为标准心衰药物疗法的补充[34-35]。

Figure 3. Mechanisms of TRPV4 contributing to pulmonary edema. ROS:
reactive oxygen species;
NO:
nitric oxide.
最近,我们课题组在小鼠主动脉弓缩窄(transverse aortic constriction, TAC)模型中观察到,激活TRPV4可通过促进心肌细胞Ca2+内流,激活CaMKII磷酸化和随后的NFκB-NLRP3途径,促进心肌炎症和纤维化,而阻断TRPV4可缓解TAC诱导的心脏肥大、心功能障碍和纤维化,表明TRPV4可成为心脏肥大和心力衰竭的治疗靶点[36]。
2.5TRPV4通道在心律失常中的作用心房颤动(atrial fibrillation, AF)是临床上常见的心律失常,其发生主要是由于心房的电重构和结构重构[37]。我们课题组率先观察到,在无菌性心包炎大鼠中,TRPV4在心房肌细胞和成纤维细胞中表达增加,口服TRPV4阻断剂GSK2193874可减少大鼠房颤的诱发率和持续时间,机制研究揭示,一方面TRPV4通过减少心房肌细胞动作电位的延长减轻心房电重构,另一方面其可通过抑制心房成纤维细胞的活化和炎症细胞的浸润减轻心房结构重构[38]。进一步,我们课题组在-/-小鼠模型中验证,阻断TRPV4可通过ERK/NF-κB信号通路抑制NLRP3炎症小体的活化,减轻心房纤维化,从而降低AF的发生[39]。由此可见,TRPV4有望作为治疗房颤,尤其是心脏外科术后房颤的靶点。
再灌注疗法是急性心肌梗死患者的标准治疗方法,然而 I/R损伤可能引发危及生命的恶性心律失常,是急性心肌梗死患者猝死的重要原因。Peana等[40]研究观察到,在老年小鼠离体心脏I/R时,TRPV4通道功能增强,介导的Ca2+内流增多,增加细胞钙超载及肌质网(sarcoplasmic reticulum, SR)钙容量,而阻断TRPV4通道改善SR钙负载和钙泄露,减少室性心律失常的发生。目前,尚不清楚抑制TRPV4的抗心律失常作用是通过对心肌细胞的影响,抑或是对心肌成纤维细胞的抗纤维化作用。未来需深入研究TRPV4在房、室心律失常发生中的作用机制。
近年来,TRPV4通道在心血管系统中的作用受到国内外研究者越来越多的重视。大量研究表明,TRPV4在血管内皮细胞和心肌细胞中表达丰富,可作为心血管疾病潜在治疗靶点,具有广泛的治疗前景。
由于TRPV4的全身激活会引起肺水肿和急性循环衰竭,故TRPV4激动剂的发展滞后于阻断剂,但如果开发新型TRPV4激动剂用来局部给药或可绕过全身性TRPV4激活带来的安全问题,可为TRPV4激动剂作为治疗提供一种独特的方法。与TRPV4激动剂相反,TRPV4的大部分治疗兴趣都集中在通道阻断剂上。目前的临床研究提示,TRPV4阻断剂在治疗充血性心力衰竭显示出良好的应用前景。但TRPV4阻断剂仍存在一些亟待解决的问题,一方面,已有的TRPV4阻断剂在大多数情况下特异性和选择性较差,且不能满足口服需要。另一方面,TRPV4 阻断剂研发尚处于初始阶段,临床报道较少。未来,寻找更为安全有效TRPV4通道阻断剂,更为精准靶向特定器官和细胞的方法可能会为TRPV4从基础向临床转化带来曙光。
[1] Inoue R, Jensen LJ, Shi J, et al. Transient receptor potential channels in cardiovascular function and disease[J]. Circ Res, 2006, 99(2):119-131.
[2] Darby WG, Grace MS, Baratchi S, et al. Modulation of TRPV4 by diverse mechanisms[J]. Int J Biochem Cell Biol, 2016, 78:217-228.
[3] Rosenbaum T, Benítez-Angeles M, Sánchez-Hernández R, et al. TRPV4:
a physio and pathophysiologically significant ion channel[J]. Int J Mol Sci, 2020, 21(11):3837.
[4] Kitsuki T, Yoshimoto RU, Aijima R, et al. Enhanced junctional epithelial permeability in TRPV4-deficient mice[J]. J Periodontal Res, 2020, 55(1):51-60.
[5]王斌斌, 吴琼峰, 廖杰, 等. TRPV4通道与缺血再灌注损伤的研究进展[J]. 临床心血管病杂志, 2018, 34(7):636-639.
Wang BB, Wu QF, Liao J, et al. Recent progress of TRPV4 channel and ischemia reperfusion injury[J]. J Clin Cardiol, 2018, 34(7):636-639.
[6]杨翠, 陈闽伟, 黄峥嵘, 等. TRPV4在纤维化中的作用机制[J]. 临床心血管病杂志, 2020, 36(3):215-219.
Yang C, Chen MW, Huang ZZ, et al. The mechanism of TRPV4 in fibrosis[J]. J Clin Cardiol, 2020, 36(3):215-219.
[7] Gao F, Sui D, Garavito RM, et al. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4:
functional significance and implication[J]. Hypertension, 2009, 53(2):228-235.
[8] Sonkusare SK, Dalsgaard T, Bonev AD, et al. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension[J]. Sci Signal, 2014, 7(333):a66.
[9] Ottolini M, Hong K, Cope EL, et al. Local peroxynitrite impairs endothelial transient receptor potential vanilloid 4 channels and elevates blood pressure in obesity[J]. Circulation, 2020, 141(16):1318-1333.
[10] Sul OJ, Ra SW. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-κB in lung epithelial cells[J]. Molecules, 2021, 26(22):6949
[11] Song Q, Zhang Y. Application of high-fat cell model in steady-state regulation of vascular function[J]. Saudi J Biol Sci, 2019, 26(8):2132-2135.
[12] Gao M, Han J, Zhu Y, et al. Blocking endothelial TRPV4-Nox2 interaction helps reduce ROS production and inflammation, and improves vascular function in obese mice[J]. J Mol Cell Cardiol, 2021, 157:66-76.
[13] Zhu Y, Wen L, Wang S, et al. Omega-3 fatty acids improve flow-induced vasodilation by enhancing TRPV4 in arteries from diet-induced obese mice[J]. Cardiovasc Res, 2021, 117(12):2450-2458.
[14] Zhou T, Wang Z, Guo M, et al. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels[J]. Food Funct, 2020, 11(11):10137-10148.
[15] Mao A, Zhang P, Zhang K, et al. Endothelial TRPV4-eNOS coupling as a vital therapy target for treatment of hypertension[J]. Br J Pharmacol, 2022, 179(10):2297-2312.
[16] Libby P. The changing landscape of atherosclerosis[J]. Nature, 2021, 592(7855):524-533.
[17] Xu S, Liu B, Yin M, et al. A novel TRPV4-specific agonist inhibits monocyte adhesion and atherosclerosis[J]. Oncotarget, 2016, 7(25):37622-37635.
[18] Gupta N, Goswami R, Alharbi MO, et al. TRPV4 is a regulator inlipopolysaccharide-induced exacerbation of macrophage foam cell formation[J]. Physiol Rep, 2019, 7(7):e14069.
[19] Golledge J. Abdominal aortic aneurysm:
update on pathogenesis and medical treatments[J]. Nat Rev Cardiol, 2019, 16(4):225-242.
[20] Shannon AH, Elder CT, Lu G, et al. Pharmacologic inhibition of transient receptor channel vanilloid 4 attenuates abdominal aortic aneurysm formation[J]. FASEB J, 2020, 34(7):9787-9801.
[21] Yellon DM, Hausenloy DJ. Myocardial reperfusion injury[J]. N Engl J Med, 2007, 357(11):1121-1135.
[22] Dong Q, Li J, Wu QF, et al. Blockage of transient receptor potential vanilloid 4 alleviates myocardial ischemia/reperfusion injury in mice[J]. Sci Rep, 2017, 7:42678.
[23] Wu QF, Qian C, Zhao N, et al. Activation of transient receptor potential vanilloid 4 involves in hypoxia/reoxygenation injury in cardiomyocytes[J]. Cell Death Dis, 2017, 8(5):e2828.
[24] Wu Q, Lu K, Zhao Z, et al. Blockade of transientreceptor potential vanilloid 4 enhances antioxidation after myocardial ischemia/reperfusion[J]. Oxid Med Cell Longev, 2019, 2019:7283683.
[25] 陈卓, 秦昆, 何玥颖, 等. TRPV4在心肌细胞缺氧损伤中的作用及机制研究[J]. 中国病理生理杂志, 2022, 38(7):1194-1200.
Chen Z, Qin K, He YY, et al. Role and mechanism of TRPV4 in myocardial hypoxia injury[J]. Chin J Pathophysiol, 2022, 38(7):1194-1200.
[26] Zhang S, Lu K, Yang S, et al. Activation of transient receptor potential vanilloid 4 exacerbates myocardial ischemia-reperfusion injury via JNK-CaMKII phosphorylation pathway in isolated mice hearts[J]. Cell Calcium, 2021, 100:102483.
[27] Jones JL, Peana D, Veteto AB, et al. TRPV4 increases cardiomyocyte calcium cycling and contractility yet contributes to damage in the aged heart following hypoosmotic stress[J]. Cardiovasc Res, 2019, 115(1):46-56.
[28] Adapala RK, Thoppil RJ, Luther DJ, et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals[J]. J Mol Cell Cardiol, 2013, 54:45-52.
[29] Adapala RK, Kanugula AK, Paruchuri S, et al. TRPV4 deletion protects heart from myocardial infarction-induced adverse remodeling via modulation of cardiac fibroblast differentiation[J]. Basic Res Cardiol, 2020, 115(2):14.
[30] Alvarez DF, King JA, Weber D, et al. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier:
a novel mechanism of acute lung injury[J]. Circ Res, 2006, 99(9):988-995.
[31] Hamanaka K, Jian MY, Weber DS, et al. TRPV4 initiates the acute calcium-dependent permeability increase during ventilator-induced lung injury in isolated mouse lungs[J]. Am J Physiol Lung Cell Mol Physiol, 2007, 293(4):L923-L932.
[32] Thorneloe KS, Cheung M, Bao W, et al. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure[J]. Sci Transl Med, 2012, 4(159):148r-159r.
[33] Balakrishna S, Song W, Achanta S, et al. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2014, 307(2):L158-L172.
[34] Stewart GM, Johnson BD, Sprecher DL, et al. Targeting pulmonary capillary permeability to reduce lung congestion in heart failure:
a randomized, controlled pilot trial[J]. Eur J Heart Fail, 2020, 22(9):1641-1645.
[35] Goyal N, Skrdla P, Schroyer R, et al. Clinical pharmacokinetics, safety, and tolerability of a novel, first-in-class TRPV4 ion channel inhibitor, GSK2798745, in healthy and heart failure subjects[J]. Am J Cardiovasc Drugs, 2019, 19(3):335-342.
[36] Zou Y, Zhang M, Wu Q, et al. Activation of transient receptor potential vanilloid 4 is involved in pressure overload-induced cardiac hypertrophy[J]. Elife, 2022, 11:e74519.
[37] Kornej J, Borschel CS, Benjamin EJ, et al. Epidemiology of atrial fibrillation in the 21st century:
novel methods and new insights[J]. Circ Res, 2020, 127(1):4-20.
[38] Liao J, Wu Q, Qian C, et al. TRPV4 blockade suppresses atrial fibrillation in sterile pericarditis rats[J]. JCI Insight, 2020, 5(23):e137528.
[39] Yang S, Zhao Z, Zhao N, et al. Blockage of transient receptor potential vanilloid 4 prevents postoperative atrial fibrillation by inhibiting NLRP3-inflammasome in sterile pericarditis mice[J]. Cell Calcium, 2022, 104:102590.
[40] Peana D, Polo-Parada L, Domeier TL. Arrhythmogenesis in the aged heart following ischaemia-reperfusion:
role of transient receptor potential vanilloid 4[J]. Cardiovasc Res, 2022, 118(4):1126-1137.
Progress in role of TRPV4 in cardiovascular diseases
DONG Qian, YU Kun-wu, DU Yi-mei△
(,,,,430000,)
Transient receptor potential cation channel subfamily V member 4 (TRPV4) is a non-selective cation channel that is widely expressed in the cardiovascular system. Activation of TRPV4 induces an increase in intracellular calcium concentration and plays an important role in both physiological and pathological conditions. Recent studies have revealed that TRPV4 plays an important role in many pathophysiological processes closely related to cardiovascular diseases, including regulating vasodilation and protecting the integrity of the endothelial cell barrier, etc. This review discusses the evidence and its potential mechanisms of TRPV4 in diverse responses including hypertension, myocardial infarction, cardiac remodeling, congestive heart failure-induced pulmonary edema, atherosclerosis, and arrhythmia.
Transient receptor potential cation channel subfamily V member 4;
Cardiovascular diseases;
Mechanism
R54;
R363
A
10.3969/j.issn.1000-4718.2023.02.021
1000-4718(2023)02-0373-06
2022-08-31
2022-12-13
[基金项目]国家自然科学基金青年科学基金资助项目(No. 82100339)
Tel:
027-85726462;
E-mail:
yimeidu@mail.hust.edu.cn
(责任编辑:林白霜,罗森)
猜你喜欢阻断剂纤维细胞内皮Tiger17促进口腔黏膜成纤维细胞的增殖和迁移昆明医科大学学报(2021年8期)2021-08-13滇南小耳猪胆道成纤维细胞的培养鉴定云南医药(2021年3期)2021-07-21环境中的β-阻断剂及其在污水处理中的工艺研究四川农业科技(2018年6期)2018-03-17β-受体阻断剂剂量对伊伐布雷定在心衰患者治疗中疗效的影响成都医学院学报(2016年4期)2016-03-26胃癌组织中成纤维细胞生长因子19和成纤维细胞生长因子受体4的表达及临床意义中国现代医学杂志(2015年26期)2015-12-23Wnt3a基因沉默对内皮祖细胞增殖的影响安徽医科大学学报(2015年9期)2015-12-16内皮祖细胞在缺血性脑卒中诊治中的研究进展医学研究杂志(2015年11期)2015-06-10两种制备大鼠胚胎成纤维细胞的方法比较中国当代医药(2015年33期)2015-03-01微丝解聚剂及微管阻断剂对藓羽藻细胞重建过程的影响中国海洋大学学报(自然科学版)(2014年12期)2014-02-28新鲜生鸡蛋壳内皮贴敷治疗小面积烫伤中国民间疗法(2013年8期)2013-01-25本文来源:http://www.zhangdahai.com/shiyongfanwen/qitafanwen/2023/0917/655545.html